ADDITIVE TO HYPOTHERMIC GRAFT PRESERVATION SOLUTIONS
The annual number of transplants is broadly constantly increasing in France, from 4,945 in 2011 to 5,276 in 2021. However, this still falls well short of demand. In 2020, 26,001 patients were waiting for organs1. More than 900 people on the transplant waiting list died due to the lack of available organs.
Faced with this shortage, regulatory and health authorities have authorized the use of organs from older and older donors and from donors who suffered cardiac death. Organs from such donors are very sensitive to the ischemia-reperfusion cycle.
In the USA, more than 115,000 people are waiting for transplants2. Yet, a study showed that out of 8,800 organs 540 were considered as lost3.
During a transplant of “solid” organs, the organ removed is experiencing a sudden stop of blood supply and therefore the oxygen necessary for its survival (i.e. ischemia). It is then usually placed in a 4°C preservation solution to reduce its metabolism (Figure 1).
Ischemia/reperfusion can lead to lesions due to the lack of oxygenation of the graft, and these play an important role in the risk of primary rejection of the graft by the recipient. Ischemia/reperfusion can lead to lesions due to the lack of oxygenation of the graft, and these play an important role in the risk of primary rejection of the graft by the recipient.
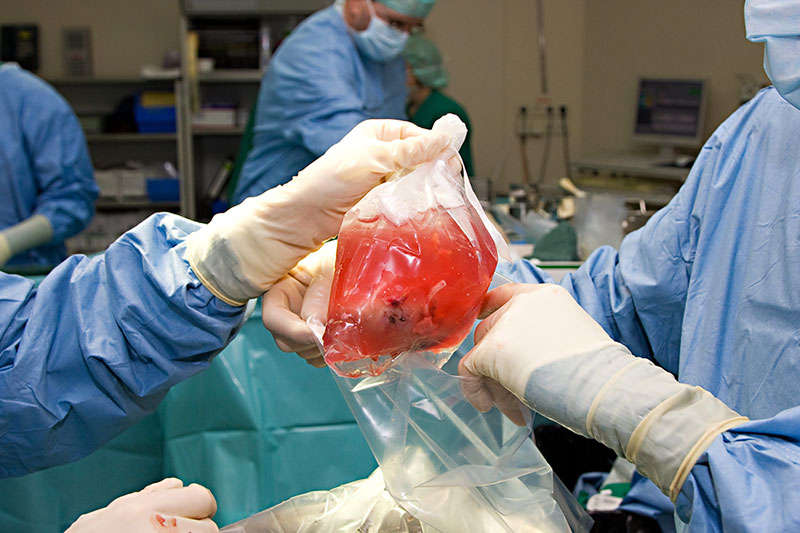


The challenge is therefore major to provide solutions to limit these lesions and thus improve the quality of the grafts.
In this context, the HEMO2life® medical device developed by Hemarina is to be used ex-vivo as an additive to preservation solutions for kidney preservation during hypothermic transplantation under static or perfused conditions.
HEMO2life® has been tested on several pre-clinical models, and has been the subject of two clinical studies in kidney transplantation (Oxyop – NCT02652520 ) led by Prof. Yannick Le Meur (CHRU Brest) and Prof. Benoît Barrou (APHP, Pitié-Salpêtrière Paris), including in total more than 550 patients.
A retrospective study (Oxyop4 – NCT05050513) has been carried out in order to follow-up Oxyop patients over 4 years. The results have showed an action on the delayed graft function and a better survival of patients who have received a graft preserved with HEMO2life®. (Zal F, 2024, International Journal of Cell/tissue Engineering, Artificial Cells & Regenerative Medicine, (accepted))
Another retrospective study, based on the comparison of Oxyop clinical data and ASTRE database containing the clinical parameters of 6,584 patients after kidney transplantation, confirmed the results of the OXYOP study and suggested that the recovery of renal function observed with HEMO2life® in the OXYOP study is due to therapeutic benefit of HEMO2life® reducing DGF independently of Cold Ischemia Time (Le Meur et al., 2022, Artificial Organ, 46, 597-605).
The Hemoglobin contained in HEMO2life® solution allows continuous physiological oxygenation of the graft through its storage and until transplantation, thereby improving graft quality by limiting ischemia/reperfusion injuries.
HEMO2 life® is a Class III Medical Device under CE mark process.
1. Biomedicine Agency, press release, CRISTAL, 03/03/2021
2. Number of candidates for transplantation in the United States, Organ Procurement & Transplantation Network
https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#
3. The Organ Shortage Crisis in America , Flescher, Andrew , Washington, DC: Georgetown University Press, 2018.
4.Long term results of the use of the oxygen carrier M101 as an additive to organ preservation solution. Zal Franck
International Journal of Cell/tissue Engineering, Artificial Cells & Regenerative Medicine, 2024 (accepted)
HEMO2life® : Related Publications
Asong-Fontem, N., Panisello-Rosello, A., Lopez, A., Imai, K., Zal, F., Delpy, E., Rosello-Catafau, J., Adam, R. A Novel Oxygen Carrier (M101) Attenuates Ischemia-Reperfusion Injuries during Static Cold Storage in Steatotic Livers Int. J. Mol. Sci. - 2021 https://pubmed.ncbi.nlm.nih.gov/34445250/
Ali, A., Watanabe, Y., Galasso, M., Watanabe, T., Chen, M., Fan, E., Brochard, L., Ramadan, K., Ribeiro, RVP., Stansfield, W., Gokhale, H., Gazzalle, A., Waddell, T., Liu, M., Keshavjee, S., Cypel, M. An extracellular oxygen carrier during prolonged pulmonary preservation improves post-transplant lung function J Heart Lung Transplant. - 2020 https://www.ncbi.nlm.nih.gov/pubmed/32334946
Le Meur, Y., Morelon, E., Essig, M., Thierry, A., Büchler, M., Drouin, S., Deruelle, C., Badet, L., Pesteil, F., Delpech, P-O., Boutin, J-M., Renard, F., Barrou, B. First-in-human use of a marine oxygen carrier for organ preservation: a safety and proof-of-principle study. Am J Transplant. - 2020 https://www.ncbi.nlm.nih.gov/pubmed/32012441
Alix, P., Val-Laillet, D., Turlin, B., Ben Mosbah, I., Burel, A., Bobillier, E., Bendavid, C., Delpy, E., Zal, F., Corlu, A., Boudjema, K. Adding the oxygen carrier M101 to a cold-storage solution could be an alternative to HOPE for liver graft preservation. JHEP Rep. - 2020 https://pubmed.ncbi.nlm.nih.gov/32695967/
Lemaire, F., Sigrist, S., Delpy, E., Cherfan, J., Peronet, C., Zal, F., Bouzakri, K., Pinget, M., Maillard, E. Beneficial effects of the novel marine oxygen carrier M101 during cold preservation of rat and human pancreas. Journal of Cellular and Molecular Medicine. - 2019 https://onlinelibrary.wiley.com/doi/full/10.1111/jcmm.14666
Kaminski, J., Hannaert, P., Kasil,A., Thuillier, R., Leize, E., Delpy, E., Steichen, C., Goujon, J.-M., Zal, F., Hauet, T. Efficacy of the natural oxygen transporter HEMO2life® in cold preservation in a preclinical porcine model of donation after cardiac death. Transplant International, official journal of the European Society for Organ Transplantation. - 2019 https://www.ncbi.nlm.nih.gov/pubmed/30924562
Thuillier, R., Delpy, E., Matillon, X., Kaminski J., Kasil, A., Soussi, D., Danion, J., Sauvageon, Y., Rod, X., Donatini, G., Barrou, B., Badet, L., Zal, F., Hauet, T. Preventing acute kidney injury during transplantation: the application of novel oxygen carriers. Expert Opinion on Investigational Drugs, 28(7):643-657. - 2019 https://www.ncbi.nlm.nih.gov/pubmed/31165652
Kasil, A., Giraud, S., Couturier, P., Amiri, A., Danion, J., Donatini, G., Matillon, X., Hauet, T., Badet, L. Individual and combined impact of oxygen and oxygen transporter supplementation during kidney machine preservation in a porcine preclinical kidney transplantation model. International Journal of Molecular Sciences, 20(8). - 2019 https://www.ncbi.nlm.nih.gov/pubmed/31018558
Glorion, M., Polard, V., Favereau, F., Hauet, T., Zal, F., Fadel, E., Sage, E. Prevention of ischemia-reperfusion lung injury during static cold preservation by supplementation of standard preservation solution with HEMO2life® in pig lung transplantation model. Artificial Cells, Nanomedicine, and Biotechnology, 46(8):1773-1780. - 2018 https://www.ncbi.nlm.nih.gov/pubmed/29069926
Ali, A., Watanabe, Y., Galasso, M., Stansfield, W., Watanabe, T., Ramandan, K., Chen, M., Ribeiro, R.P., Gokhale, H., Gazzalle, A., Waddell, T., Liu, M., Keshavjee, S., Cypel, M. Two-Day Lung Preservation Followed by Lung Transplantation in a Large Animal Model Using Novel Extracellular Oxygen Carrier. The Journal of Heart and Lung Transplantation, - 2018 https://www.jhltonline.org/article/S1053-2498(18)30296-1/abstract
Lemaire, F., Bietiger, W., Peronet, C., Langlois, A., Mura, C., Bouzakri, K., Sigrist, S., Polard, V., Zal, F., Pinget, M., and Maillard, E. Effect of HEMO2life®, a marine oxygen carrier, for pancreas preservation during cold ischemia. Poster presented during 8th international EPITA Symposium, Innsbruck, Austria. - 2018
Teh, E. S., Zal, F., Polard, V., Menasché, P., Chambers, D.J. HEMO2life® as a protective additive to Celsior solution for static storage of donor hearts prior to transplantation. Artificial Cells, Nanomedicine, and Biotechnology, 45(4):717-722. - 2017 https://www.ncbi.nlm.nih.gov/pubmed/28079401
Mallet, V., Dutheil, D., Polard, V., Rousselot, M., Leize, E., Hauet, T., Goujon, J.-M., Zal, F. Dose-Ranging study of the performance of the natural oxygen transporter HEMO2life® in organ preservation. Artificials Organs, 38(8):691-701. - 2014 https://www.ncbi.nlm.nih.gov/pubmed/24749976
Zal, F., Rousselot, M. Extracellular Hemoglobins from Annelids, and their Potential Use in Biotechnology Outstanding Marine Molecules, Wiley‐VCH Verlag GmbH & Co. KGaA, - 2014 https://onlinelibrary.wiley.com/doi/abs/10.1002/9783527681501.ch16
Thuillier, R., Dutheil, D., Trieu, M.T.N., Mallet, V., Allain, G., Rousselot, M., Denizot, M., Goujon J.-M., Zal, F., Hauet, T. Supplementation with a new therapeutic oxygen carrier reduces chronic fibrosis and organ dysfunction in kidney static preservation. American Journal of Transplantation, 11(9):1845-1860. - 2011 https://www.ncbi.nlm.nih.gov/pubmed/21875432
Le Meur, Y., Delpy, E., Renard, F., Hauet, T., Badet, L., Rerolle, JP., Thierry, A., Büchler, M., Zal, F., Barrou, B. HEMO2life® improves renal function independent of cold ischemia time in kidney recipients: A comparison with a large multicenter prospective cohort study Artificial Organs. - 2021 https://pubmed.ncbi.nlm.nih.gov/34951495/
Prof Laurent Lantieri, MD, Prof Bernard Cholley, MD, Prof Cedric Lemogne, MD, Romain Guillemain, MD, Prof Nicolas Ortonne, MD, Prof Philippe Grimbert, MD, et al First human facial retransplantation: 30-month follow-up The Lancet - 2020 https://www.thelancet.com/article/S0140673620324387/abstract
Latest press releases
Hemarina and Delpharm announce the signing of a partnership for the manufacture of HEMO2life®
November 29, 2018
Brest University Hospital and HEMARINA present the new positive advances of the first clinical trial of HEMO2life® at the American Transplant Congress in Seattle,
June 6, 2018
Hemarina and the University Health Network of Toronto announce positive preclinical proof of concept results for the HEMO2life® oxygen transporter in the preservation of lung transplants before transplantation
April 12, 2018
Brest University Hospital and HEMARINA announce positive results from the first clinical trial of HEMO2life® in kidney conservation before transplantation in humans.
November 14, 2017

