Hemarina
A human, scientific, and entrepreneurial adventure




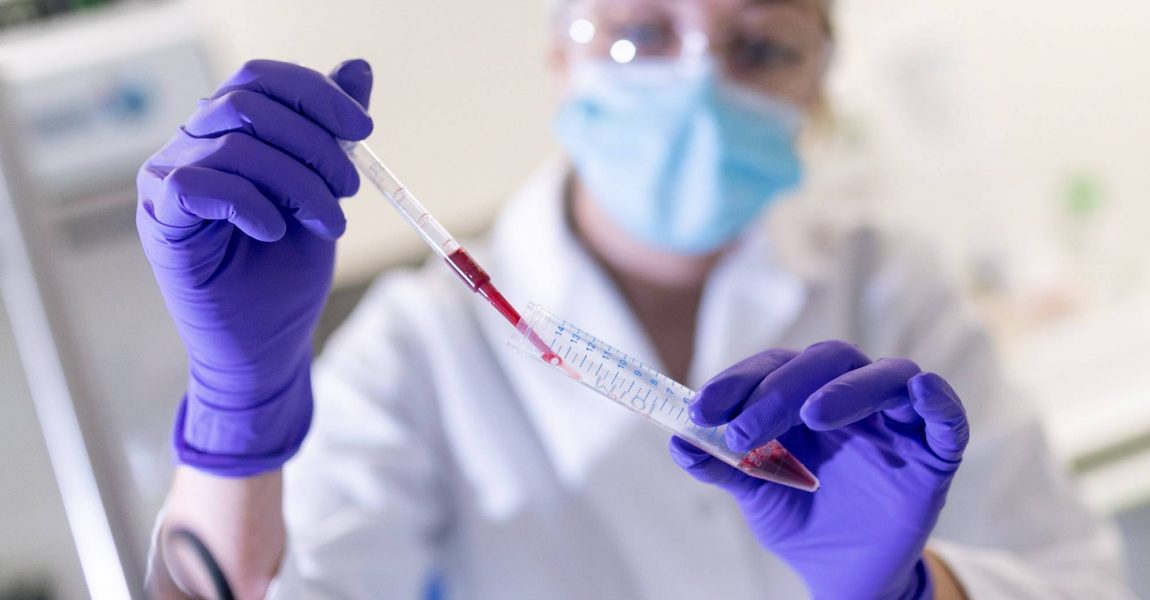





Our history
Hemarina is a biopharmaceutical company specialized in the development of health products via its proprietary technology platform (M101) based on the properties of Lugworm (Arenicola marina) hemoglobin. A spin-off of the CNRS and Sorbonne University (Paris VI), Hemarina was created in 2007 in Northern Finistère.
Convinced that this natural biopolymer had potential as an innovative therapeutic option to address diseases for which there are significant unmet medical needs, Franck Zal created his company based on fifteen years of fundamental research.
A major innovation compared to all the other current Hemoglobin-Based Oxygen Carriers (HBOCs) Hemarina’s unique technology in the world is based on a naturally occurring extracellular hemoglobin of high molecular weight, that is functional over a wide temperature range (from 4°C to 37°C) and does not require any cofactor to release oxygen. To date, this is the only known hemoglobin with these properties.
One of the essential advantages of this technology is the absence of vasoconstrictor and hypertensive effects as observed with first and second generation HBOCs manufactured from animal (bovine or porcine) or human hemoglobin.

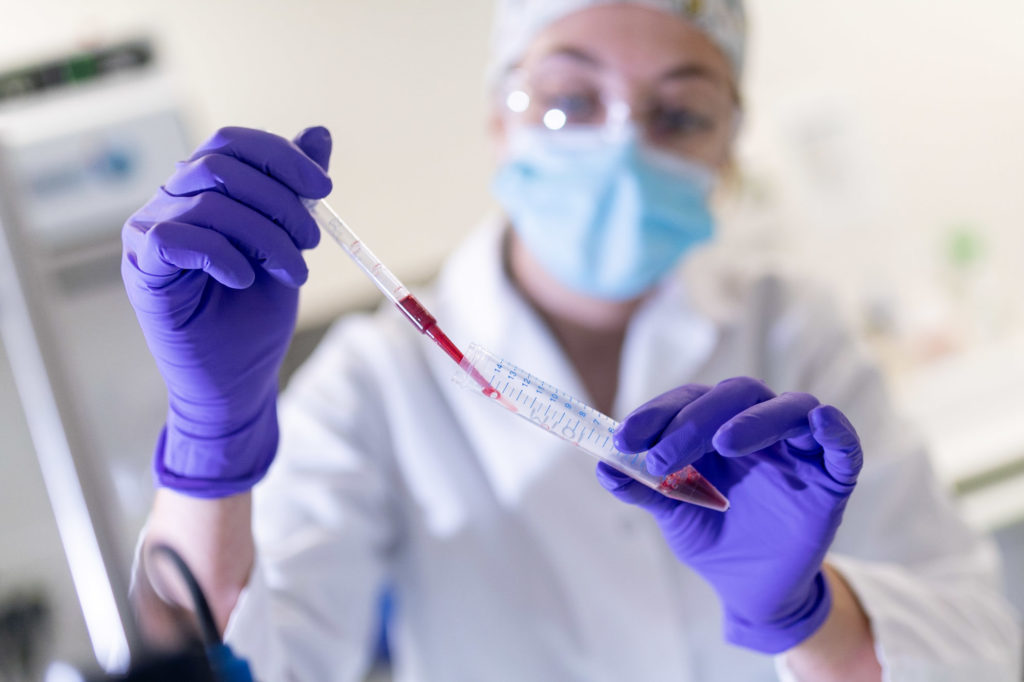
PROMISING APPLICATIONS
The prospects for therapeutic applications are therefore immense. Why? Quite simply because this molecule is a technological platform; it can be used wherever oxygen is involved and potentially at all levels of the living organism (cell, tissue, organ and organism). As a result, all ischemic diseases (resulting from low blood flow and oxygen deficiency) are potential pathways for development.
The heart, kidney and brain are the most sensitive organs to oxygen deficiency. Therefore, these organs are the targets of technology development at Hemarina. Ischemia can result in heart failure, myocardial infarction, and stroke.
Another objective of Hemarina’s technology is to help blood banks meet the growing need for red blood cells. Currently, legal requirements state that human blood cells can be stored for a maximum of 42 days between +2° and +6°C. We are developing a freeze-drying system that will extend the storage life of our red blood cell-equivalent product for 5 years at room temperature.
This freeze-dried therapeutic oxygen carrier will be the first of its kind, and of major importance in the health sector. It will allow rapid use without a regulated storage chamber (equipment for SAMU vehicles, for first aid care) and no thawing constraints, thus providing an optimal solution for the treatment of crises.
Our current efforts focus on the development of the following health products:
- HEMO2life®: This medical device is an additive to graft preservation solution, Class III Medical Device and has already earned the support of the scientific and medical community.
- HEMHealing®: an oxygenating dressing
- HEMOXYCarrier®: a universal therapeutic oxygen carrier (similar to red blood cells).
- HEMDental-Care®: a treatment for periodontal diseases.
- HEMOXCell®: a cell growth activator
The novelty and potential of these solutions have been recognized through numerous international collaborations with academic, clinical and pharmaceutical partners in Europe and the United States.
The results of this research have been published in numerous peer-reviewed scientific journals and protected by patents, thus forming the foundations of Hemarina’s technology.
OUR VALUES
Patients are at the heart of all our actions. Our ultimate objective is to provide solutions that contribute to improving the quality of life of patients and their families.
Continuous innovation for diseases with unmet medical needs is a priority.The objective is to provide maximum benefit with minimal changes in medical practices, thereby limiting the cost to the health system.
Main prizes and awards
2023
2019
- Winner of the Prix Galien France in the medical device category for HEMO2life®
- Winner of the Europe 1 Award for the Future of Health - 02/2019
2018
- Prize for the innovative company 2018, Les Victoires de la Bretagne, Le Télégramme.
- Start-up of the Year; Regional and national EY Entrepreneur of the Year Award. This award recognizes a high-potential start-up leader who has demonstrated innovation in his market and is a driving force for French growth and competitiveness.
- Maritime Innovation Award: Trophy of the Breton conquerors.
- Unicorn Trophy Nominee 2018: HEMARINA distinguished among the 10 most promising French companies in the Biotech/Medtech sector at the Future Unicorn Awards. This award recognizes privately-held French technology companies that show remarkable growth prospects and show potential to become a “Unicorn”, i.e. technology companies whose value exceeds €1 billion.
2017
- Créative Next Prize from Business France in New York.
- 2nd CCI France International Trophy.
2016
- Prix Galien Medstartup This prize rewards the partnership between French start-ups/SMEs (Hemarina) and American health companies (Naval Medical Research Center, US Navy) for the development of breakthrough innovations in the field of Bio- and Medical technology.
- Hemarina named SME of the Year at the French American Business Awards ceremony. Awarded by the FACCNE, the French-American Chamber of Commerce of New England.
2015
- Fast 50 Prize
- First Prize of the AGBM (Alliance for Biological and Medical Engineering) for Innovative Medical Technologies. Prize awarded by Professor Carpentier for HEMO2life®.
- “Grandes Ecoles" Trophy of the Business Angels of the Grandes Ecoles.
2013
- Prize for Bio-tech of the Future – from Région Ouest.
2012
- E&Y Born Global Award – From Région Ouest.
2011
- Prize for innovative health entrepreneur From Health Business Angels.
2007
- Winner of the Senators Grand Prize in the Life Sciences category at the Tremplin Entreprise event.
- Winner of the Start West Award for Business Creation awarded by the Region of Brittany.


